Abstract
Background: High dose chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard of care therapy for patients with newly diagnosed multiple myeloma. Daratumumab, an anti-CD38 monoclonal antibody, is now frequently being incorporated as a part of induction therapy in clinical practice, on the basis of data from several clinical trials.1-3 Few studies have evaluated the impact of daratumumab on stem cell mobilization and engraftment with conflicting results. Delayed neutrophil engraftment was noted in patients who received daratumumab as a part of induction therapy in one study.4 However, no delay was reported in another retrospective study5 and at least one clinical trial.6 Lower numbers of stem cells collected were reported uniformly in all three studies with daratumumab use. We evaluated the impact of daratumumab on stem cell yield with mobilization therapy as well as engraftment kinetics.
Methods: We retrospectively evaluated 353 patients with newly diagnosed multiple myeloma treated at Hackensack University Medical Center between 2019-2021, on an IRB approved protocol. As per institutional guidelines, a combination of granulocyte colony stimulating factor (GCSF) with plerixafor was used as the mobilizing regimen for patients >70 yrs; cyclophosphamide, etoposide and dexamethasone (CdE) with GCSF was used for patients <70. Plerixafor could be added after chemotherapy mobilization for patients failing to achieve the desired collection goals. All patients received melphalan at a dose of 200mg/m2 administered intravenously on day -1. Time to engraftment (TTE) was calculated from the day of stem cell infusion to neutrophil and platelet engraftment, each defined as an absolute neutrophil count (ANC) of over 500/uL for three sequential tests and a platelet level over 20,000/uL without transfusion support, respectively. Goal for stem cell collection was defined as >8 x 106 CD34+ cells for patients >65 years, >12 x 106 CD34+ cells for patients <65. Statistical analyses were performed using STATA v16.0 (Stata Corp, TX, USA). The differences in median times to engraftment were analyzed using the Wilcoxon signed-rank test. Neutrophil and platelet recovery over time were calculated and plotted using Kaplan-Meier survival analyses and log-rank test for equality of survival function. Differences were considered significant at p-values <0.05.
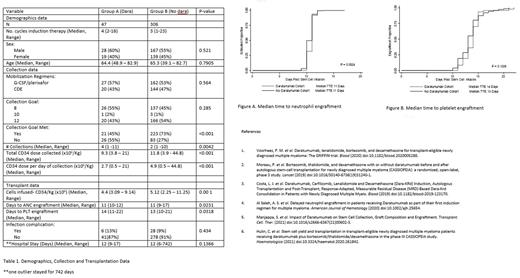
Results: 47 patients received daratumumab as a part of their induction therapy (Group A) compared with 306 patients who did not (Group B). Both groups were well balanced with regards to number of cycles of remission-induction therapy, age, sex, and mobilization regimens (Table 1). A higher number of CD34+ cells were collected for Group B, compared with Group A (12 x 10^6 vs 9.5 x 10^6, p<0.001); a lower number of collection days were required to reach collection goal for Group B compared with Group A (2 days vs 3 days, p=0.01); more CD34+ cells were collected per day for Group B compared with Group A (4.9 x10^6 vs 3.0 x 10^6, p=0.01). 73.5% patients met their collection goal for Group A, compared with 46% for Group B (p<0.001). A second mobilization attempt was required for 7 (14.9%) of patients in Group A compared to 10 (3.3%) for Group B. No difference was noted with regards to TTE for ANC (11 days for both groups, p=0.005) (Figures A); TTE for platelets was noted to be delayed by 1 day for Group A (14 days vs 13 days, p=0.0168) (Figures B). Rates of bacteremia did not differ between the two groups. Results are summarized in Table 1.
Conclusions In our single-center study, a lower number of CD34+ cells were collected for patients who received daratumumab as a part of induction therapy for myeloma despite the use of standard mobilization regimens. No difference was found with regards to ANC engraftment. Platelet engraftment occurred one day earlier for patients who did not receive daratumumab for induction. These observations are consistent with results published in two prior studies.5,6 Depending on the target goal for CD34+ cell collection, daratumumab should be used with caution in the upfront setting for multiple myeloma. This is particularly important if tandem stem cell transplantation, salvage transplantation, or stem cell boost post high dose therapy are being considered as prospective treatment options for patients with multiple myeloma.
Disclosures
Rowley:SIRPant Immunotherapeutics: Consultancy; ReAlta Life Sciences: Consultancy. Biran:BMS: Consultancy, Research Funding; Merck: Research Funding; Karyopharm: Research Funding; Amgen: Research Funding; Janssen: Consultancy, Research Funding; Sanofi: Consultancy; Abbvie: Consultancy. Vesole:Takeda: Speakers Bureau; BMS: Speakers Bureau; GSK: Speakers Bureau; Karyopharm: Speakers Bureau; Amgen: Speakers Bureau; Jassen: Speakers Bureau. Siegel:COTA: Current equity holder in private company, Current holder of stock options in a privately-held company; BMS: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Takeda: Honoraria; Janssen: Honoraria; Merck: Honoraria; Celularity: Membership on an entity's Board of Directors or advisory committees. Parmar:Janssen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.